1. INTRODUCTION
Cajuput (Melaleuca cajuputi Powell) is a plant that has been managed for a long time in Indonesia, especially by Perum Perhutani in Java. Cajuput oil production in Indonesia reached 25.06 million liters in 2020, an increase of 229.50% compared to the previous year (BPS, 2020). Given its considerable contribution to the company’s income, increasing cajuput oil production as a non-timber forest product has been a priority for Perum Perhutani in recent years. To this end, the cajuput plantation area has expanded significantly in West, Central, and East Java. However, in line with the cajuput plantation extension, subterranean termite infestation of the seedlings increased significantly.
According to Perhutani’s Research and Development Center and the Faculty of Forestry of the IPB (2019), the area of cajuput plants attacked by subterranean termites is estimated to reach 1,295.60 ha. Subterranean termite attacks on cajuput plants have caused significant economic losses for Perum Perhutani because the company incurs additional costs for replanting dead termite- infested plants and controlling these pests, as well as indirect losses due to delays in planting.
Subterranean termite attacks in the Inhutani area have occurred for a long time. Arif et al. (2021) reported that the subterranean termites Coptotermes curvignathus and Oryzias javanicus attack a logged-over area planted with M. cajuputi. The Perhutani’s Research and Development Center and the Faculty of Forestry of IPB (2019) also reported that cajuput seedlings in the Forest Management Units Kuningan and Semarang were attacked by the subterranean terimite Macroternes gilvus with attack frequencies of 14.85% and 15.39%, respectively. It was also reported that among the infested seedlings, 94.20%–97.09% experienced attacks with an intensity classified as severe, including death.
Termite attacks target the roots of plants. This part of the plant is suspected to contain chemical compounds that are attractants for subterranean termites. If this assumption is correct, cajuput seedling roots could potentially be a source of termite attractants that can be utilized as essential elements for controlling subterranean termite attacks on cajuput plants. Subterranean termite attacks on cajuput seedling roots indicated that they were attracted to these parts of the plant.
Currently, the total area for planting cajuput is 53,394 ha, with the realization of Cajuput oil product revenue of IDR 17.83 billion or 13.13% of the 2022 Company’s Work Plan and Budget, an 81.99% decrease in revenue compared to revenue in the same period in 2021 (Perhutani, 2022). Therefore, the use of other cajuput plants needs to be studied to increase state revenue from processed derivatives of cajuput by utilizing cajuput seedling root extracts as attractants for controlling subterranean termites.
Therefore, it is necessary to study the potential of cajuput seedling root extract as an attractant of C. curvignathus subterranean termites. Previous studies have shown that eucalyptus leaf extract contains eugenol and sineol compounds that have potential as attractants (Indrayani et al., 2016). This study aimed to characterize the root extract of cajuput seedlings (M. cajuputi) and determine its bioactivity as an attractive candidate for the subterranean termite C. curvignathus.
2. MATERIALS and METHODS
The materials used in this study included cajuput seedling roots (including the base), n-Hexane, distilled water, sand, Whatman no.1 filter paper, and the subterranean termite (Coptotermes curvignatus Holmgren). The tools used in the study included gas chromatography- mass spectrometry (GC-MS; Shimadzu GC-MS QP 2010), a socklet, water bath, Wiley mill, filter, Erlenmeyer flask, oven, electric balance, desiccator, plastic net, H-tube, and jar bottle.
Cajuput seedlings used in this study were seedlings of Clone 59, which were ± three months old, originating from the nursery of Perum Perhutani Forest Management Unit Banten, Banten Province, as many as 60 seedlings. Cajuput seedlings were grown in polybags with a diameter of 7.50 cm and a height of 20 cm.
Cajuput seedling roots were separated from the rest of the plants and dried to 12.00% moisture content. The roots were then ground using a Willey mill and filtered through a sieve into a 40–60 mesh powder.
Cajuput seedling root extraction was performed according to the ASTM D 1108–96 standard with n-Hexane as the solvent (American Society for Testing and Materials, 2001). A total of 2 g of cajuput seedling root powder was placed in a socklet and extracted with 150 mL of n-Hexane for 6–8 hours. After the powder was evaporated from the solvent, the powder was then dried in an oven at 100°C–105°C for 1 h. The powder was then placed in a desiccator and weighed until the weight remained constant. First, 2 g of the cajuput seedling root powder was weighed for moisture content testing. The test samples were dried in an oven at 100°C–105°C for 2 hours. The test sample was then placed in a desiccator and weighed to determine water content. The formula for calculating solubility in n-Hexane is as follows:
Description:
ω1: Weight of the test sample (g).
ω2: Weight of the test sample after extraction (g).
p: Moisture content of the test sample (%).
The extract was prepared by dissolving the evaporated crude extract in an n-Hexane solvent. The extract solutions were prepared at three concentrations: 0.50%, 0.75%, and 1.00% (w/v). In addition, a control solution (distilled water without the cajuput seedling root extract) was prepared.
Bioactivity testing of the cajuput seedling root extract solution was performed using the attractiveness bioassay method to determine the attractiveness of the extract solution for C. curvignathus subterranean termites. In addition, toxicity testing of the extract solution against C. curvignathus was performed using a no-choice feeding bioassay. The attractiveness bioassay testing procedure followed Abdullah et al. (2015) and Indrayani et al. (2017), whereas the no-choice feeding bioassay test procedure followed 7207:2014 (Badan Standardisasi Nasional, 2014), modified from Hikma et al. (2012) and Syafii (2000). The bioassay test media consisted of two vertical glass tubes (inner diameter: 5 cm, outer diameter: 5 cm, height: 12 cm) connected to a horizontal glass tube (inner diameter: 1.5 cm, outer diameter: 2 cm) at each base. The construction of these tubes is called the “H-tube” (Fig. 1).
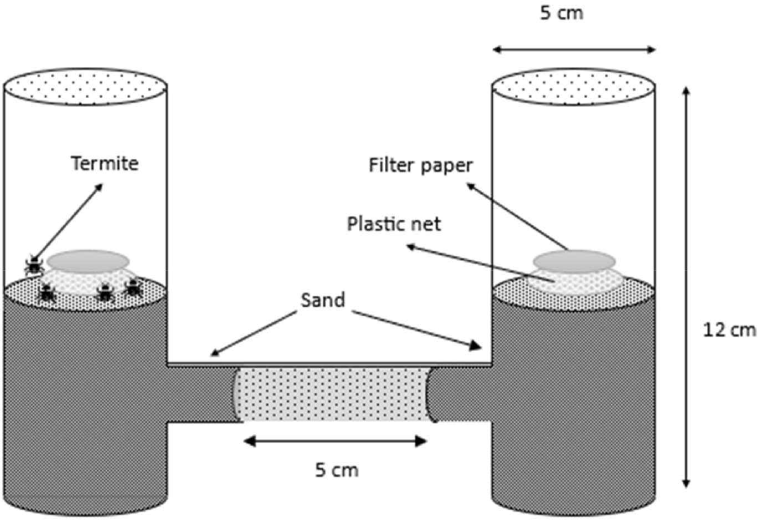
Each vertical tube contained 50 g of sand. A plastic net and filter paper (with and without treatment) were placed inside each vertical tube. The sand was moist (30–40 mL of water per 200 g dry sand). Next, 50 subterranean termite workers and five soldiers were inserted into vertical tubes containing filter paper without extract (without treatment). Tube H was then stored in a dark room (temperature 25.70 + 0.40°C and 87.50 + 0.40% relative humidity). The evaluation was conducted for 6 h in triplicates. At the end of the test, the number of termites in the vertical tube containing the treated extract was counted. To determine whether the extract could be declared as an attractive activity, an attractiveness index (AI) analysis was conducted, referring to Kramer and Mulla (1979). AI ranges from –1 (non- attractant) to 1 (attractant). The AI is calculated using the following formula:
Description:
Nt: Number of termites in a vertical tube containing filter paper with the treatment/extract.
Nc: Number of termites in vertical tubes containing filter paper without treatment/extract.
The no-choice feeding bioassay test was conducted on media in the form of a jar bottle with a diameter of 6.178 cm (outside) and 5.315 cm (inside), and a height of 12 cm (Fig. 2). The bottle was then filled with 50 g of moist sand (30–40 mL of water per 200 g), a plastic net, and filter paper (with/without treatment). Forty-five workers and five soldiers of C. curvignathus termites were placed in a jar. All jar bottles were then put in a dark room with a temperature of 25.70 + 0.40°C and relative humidity of 87.50 + 0.40% for seven days with three alternations. At the end of the test, C. curvignathus termite mortality was calculated using the following formula:

The Median Lethal Concentration (LC50) of the cajuput seedling root extract solution was determined using probit analysis as described by Finney (1971). The database used was the number of termites that died at the end of the observation period (7 d) at each solution concentration. The LC50 indicates the toxicity of the extract to termites and is classified into several classes (Table 1) based on Nguta et al. (2012).
| LC50 (μg/mL) | Description |
|---|---|
| LC50 < 100 | Strong cytotoxic activity |
| 100 < LC50 < 500 | Moderate cytotoxic activity |
| 500 < LC50 < 1,000 | Low cytotoxic activity |
| LC50 > 1,000 | Non-toxic |
Data from Nguta et al. (2012).
Extracts of the stem base and cajuput seedling roots were analyzed for chemical content using pyrolysis gas chromatography-mass spectrometry (Py-GCMS; Shimadzu GC-MS QP 2010). The solid extracts were used as samples. The extract (without preparation) was injected into nature, which was then heated in an oxygen-free environment at 280°C. Heating breaks the chemical bonds in the macromolecular structures and produces specific macromolecules. The compound mixture then entered the analysis column with a size of 60.00 mm × 0.25 mm × 0.25 μm. The carrier gas used was helium gas. The pressure used was 101 kPa with a total and column flow of 46.5 mL/min and 0.85 mL/ min. The data obtained was analyzed using GC-MS data analysis and was matched with the database Willey 9th Library.
The study was arranged in a completely randomized design with three concentrations of the extract solution (0.50%, 0.75%, and 1.00%) as the treatments. If the variance analysis (ANOVA) results showed a significant difference between treatments, it was further tested using Duncan’s test.
3. RESULTS and DISCUSSION
The results showed that the average yield of cajuput seedling roots extracted with n-Hexane was 4.94%. According to Jasni et al. (2016), the yield obtained was relatively high above 4.00% (Table 2). The yield was strongly influenced by the ability of the solvent to dissolve the extractive substances present in the material. Solvent solubility influences the ability of a solvent to dissolve a compound (Leksono et al., 2018; Mariana et al., 2018). The main principle of the extraction process is the dissolution of compounds (extractive substances) with the same polarity (Verdiana et al., 2018). Polar solvents dissolve polar compounds, whereas nonpolar solvents dissolve nonpolar compounds.
| Wood type | Composition class (%) | ||
|---|---|---|---|
| High | Moderate | Low | |
| Hardwood | 4 | 2–4 | 2 |
| Softwood | 7 | 5–7 | 5 |
The yield indicates the ability of the solvent to dissolve the compounds contained in the material. The higher the yield obtained, the better is the solvent that dissolves the compounds contained in the material. Hasanah et al. (2016) reported that n-Hexane could dissolve steroid compounds. In addition, n-Hexane can dissolve compounds such as fats, waxes, fixed oils, and terpenes (Nkogo et al., 2022). The extraction yields are not only influenced by the polarity of the solvent used; Frankó et al. (2018) stated that extraction results are influenced by other factors as well, such as temperature, time, and particle size.
The results showed that the attractiveness of cajuput seedling root extract solutions at concentrations of 0.50%, 0.75%, and 1.00% for C. curvignathus termites was 45.33%, 62.00%, and 74.67%, respectively (Fig. 3). The results of variance analysis (α = 0.05) showed that the concentration had a significant effect on the number of termites attracted (p < 0.05). Duncan’s test results (Table 3) showed that the extract solution at a concentration of 1.00% was significantly different from the 0.50% extract but not significantly different from the 0.75% extract.

| Concentration (%) | N | Subset | |
|---|---|---|---|
| 1 | 2 | ||
| 0.50 | 3 | 45.33 | - |
| 0.75 | 3 | - | 62.00 |
| 1.00 | 3 | - | 74.67 |
| Sig. | - | 1.00 | 0.057 |
The attractiveness of an extract was determined using its AI. The AI can show whether an extract is a repellant or attractant. Alves et al. (2018) stated that the AI value ranges from –1 to 1. Negative values indicated that the extract was a repellant, whereas positive values indicated that the extract was an attractant.
Table 4 shows the AI values of cajuput seedling root extract at concentrations of 0.50%, 0.75%, and 1.00% are –0.09, 0.24, and 0.49, respectively. Based on this, only extracts with concentrations of 0.75% and 1.00% were classified as attractants, whereas extracts with a concentration of 0.50% were classified as repellants. Indrayani et al. (2018) stated that the attractivity of the extract solution is related to the type and concentration of chemical compounds contained in the extract. There was a positive relationship between the type and concentration of chemical compounds and the attractivity of the extract solution. Fig. 3 shows that the higher the termite concentration, the greater the number of termites attracted. The results of research by Indrayani et al. (2017) and Septiana and Husni (2017) prove that the higher the extract concentration used, the greater the attractivity of the extract solution. The effect of concentration on attractivity is also supported by Tascioglu et al. (2012), who stated that concentration influences retention. Retention is closely related to the amount of material (chemical compounds) that constitutes the concentration.
| Concentration (%) | Nt | Nc | AI |
|---|---|---|---|
| 0.50 | 22.67 | 27.33 | –0.09 |
| 0.75 | 31.00 | 19.00 | 0.24 |
| 1.00 | 37.33 | 12.68 | 0.49 |
Research on the activity of plant extracts against termites has been previously conducted. Indrayani et al. (2018) examined the attractiveness of five plant types (bay leaves, cloves, eucalyptus, basil, and cinnamon). This study found more termites on filter paper treated with clove, eucalyptus, and cinnamon leaf extracts than on filter paper that was not given extracts, and was inversely proportional to filter paper treated with basil and bay leaf extracts. In another study, Indrayani et al. (2017) stated that eucalyptus leaf extract with 10% and 50% can attract C. curvignathus ± 80% subterranean termites, much higher than the results obtained. Presumably, the difference in results was due to the different concentrations used. Roszaini et al. (2013) reported that the activity of essential oils from Cinnamomum camphora, Cymbopogon nardus, M. cajuputi, and Dipterocarpus sp. against the subterranean termite C. curvignathus showed that M. cajuputi had the lowest mortality rate, with higher filter paper consumption than the other essential oils. Indrayani et al. (2017) used a much higher concentration than that used in this study. Indrayani et al. (2017) stated that there are eugenol compounds in clove, cinnamon, and eucalyptus leaf extracts, as well as eugenol derivatives (methyl eugenol). Therefore, eugenol and its derivatives influenced the activity of the extract. Extracts from M. cajuputi have been reported to exert a wide range of biological effects in vitro and in vivo. Phenolic, aromatic, flavonoid, and alcohol compounds exhibit antimicrobial, antioxidant, and toxic properties (Isah et al., 2023).
Several studies have reported the attractiveness of organic materials for termites. Ameka et al. (2022) reported that variations in wood chemistry influence termite feeding and substrate preferences. The plants that attract the most termites are Grevillea, Eucalyptus, and Cypress. Phytochemical analysis has shown that eucalyptus contains alkaloids, carbohydrates, cardiac glycosides, flavones, phenols, saponins, resins, and tannins. Nisar et al. (2022) reported that Conocarpus lancifolius extracts using water, methanol, and chlorfenapyr solution with methanol showed maximum repellency and were lethal against fungus-growing subterranean termites. Iqbal et al. (2018), in their research using Bombax malabaricum, Ficus religiosa, and Populus euramericana wood, concluded that these woods could be efficiently utilized at bait stations to maximize the efficacy of baiting against Odontotermes obesu. In addition, Achmad et al. (2021) reported that pineapple peel extract with methyl eugenol could attract termites to eat bait. The addition of citric acid can also increase the termite-feeding activity, as shown by the increase in the weight loss of the samples used (Priadi et al., 2023). It is best to add dye markers to observe termite attractiveness because the impact on termite mortality is not very significant when compared to controls at a concentration of no more than 2% (Im and Han, 2020).
The results showed that the mortality of C. curvignathus termites at the end of the observation period varied between the concentrations. The mortality of C. curvignathus termites after exposure to the extract solution ranged from 3.70% to 24.44% (Fig. 4). The results of variance analysis (α = 0.05) show that concentration affects termite mortality (p < 0.05). The Duncan’s test results showed that the extract at a concentration of 1.00% was significantly different from the other extract concentrations (Table 5).

| Concentration (%) | N | Subset | |
|---|---|---|---|
| 1 | 2 | ||
| 0.00 | 3 | 3.70 | - |
| 0.50 | 3 | 9.63 | - |
| 0.75 | 3 | 10.37 | - |
| 1.00 | 3 | - | 24.45 |
| Sig. | - | 0.07 | 1.00 |
Termite mortality was strongly influenced by the concentration of the extract and the type of toxic compounds present in the extract. Fig. 4 shows that the greater the concentration, the more termites died. Indrayani et al. (2016) stated that termite mortality was more significant in 0.10% eucalyptus leaf extract than in 0.01% extract (36.80% vs. 18.40%). In addition, Alshehry et al. (2014) showed that the greater the concentration of the extract used, the greater the mortality of termites obtained from each type of plant used. Based on this, increasing the concentration of the extract used will have implications for increasing the mortality or death of termites. High extract concentrations, apart from increasing the attractiveness of subterranean termites, can cause high mortality if the extractive substances are toxic to subterranean termites. Previous studies have reported that vinegar wood from durian and Nipah Fruit increased mortality in subterranean termites in proportion to the high concentration (Arsyad et al., 2020; Oramahi et al., 2022; Suprianto et al., 2023).
The type of compound also affects termite mortality. The effect of the compound type is related to the solvents used. This is thought to be related to the type of chemical compounds dissolved in the solvents. Extractive substances are one of the compounds that affect termite mortality. Kadir (2017) stated that phenolic groups are extractive substances that are toxic to termites. One type of phenolic compound is eugenol (Mu’nisa et al., 2012; Prabawati and Agustina, 2015).
Eugenol is one of the chemical compounds known to be toxic to termites (Indrayani et al., 2016; Roszaini et al., 2013). Eugenol compounds are found in various types of plants such as cloves, cinnamon, bay leaves, and eucalyptus. Eugenol is an ingredient with antibacterial properties (antibacterial activities). Nejad et al. (2017) stated that eugenol could inhibit the growth of several gram-negative (Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa) and gram-positive (Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus) bacteria. In addition, Xie et al. (2015) reported that eugenol and several other compounds with the same chemical structure (methyl eugenol, acetyl eugenol, isoeugenol, methyl isoeugenol, and acetyl isoeugenol) resulted in high mortality rates after seven days of feeding.
The toxicity of eugenol in termites is closely related to the endosymbionts present in the termite gut. Subekti and Saniaturrohmah (2020) explained that eugenol affects termite endo-symbionts (bacteria) and changes the permeability of bacterial cell membranes, resulting in the death of these bacteria. This has implications for termite death, because termites cannot digest the food they eat. Termites lose assistance in degrading food sources (cellulose) carried out by endosymbionts (bacteria). Lee et al. (2020) reported that Borneolum syntheticum,Ephedra sinica, and menthol extracts can inhibit enzyme activity in the termite gut, which can cause termite death and reduce termite feeding activity by up to 72.96%. In addition to eugenol, other compounds in the phenol, ketone, benzene, and aldehyde groups are known to have strong anti-termite properties (Hadi et al., 2020).
Environmental conditions affect termite mortality. Hasman et al. (2019) reported that unfavorable environmental conditions can cause termite death. Previous research stated that the research results showed that the average air temperature at the research location was 25.15 ± 1.46°C with humidity 75%–90%, indicating optimal termite feeding behavior, especially for subterranean termites from the Termitidae and Rhinotermitidae families such as Macrotermes sp., Microtermes sp., Capritermes sp., Schedorhinotermes sp., and C. curvignathus (Arinana et al., 2022; Hadi et al., 2018). In addition, Tampubolon et al. (2015) explained that in addition to environmental conditions, the nature of cannibalism in termites also affects termite mortality. Cannibalism appears to function in maintaining efficiency and energy conservation principles and in regulating the homeostatis (balance of life). These factors significantly affected termite mortality.
The results showed that the regression equation was y = 2.0449x – 3.9759 (Fig. 5). The LC50 value of cajuput seedling root extract is 2.45% or 24,510 μg/mL (Table 6). Kadir (2017) stated that the LC50 value indicates the toxicity of the material. The lower the LC50 value, the more toxic the material. A low LC50 value indicates that a small amount of the material used (extract) can cause 50% mortality in termites. Shanbhag and Sundararaj (2013) reported that phenolic compounds are toxic. The high phenolic compound content in the extract has implications for the low LC50 values. This proved that the type of compound and its amount (content) affected the LC50 value or toxicity of the extract. Based on the classification of Nguta et al. (2012), the n-Hexane extract solution is classified as nontoxic (non-toxic) because the LC50 value is more than 1,000 μg/mL. Roszaini et al. (2013) stated that the LC50 value of cajuput wood oil is 4.60% or 46,000 μg/ mL and is classified as less effective as an anti-termite compared to other plant extracts such as C. camphora, C. nardus, and Dipterocarpus sp.
| Solvent | Regression equation | LC50 (μg/mL) |
|---|---|---|
| n-Hexane | y = 2.0449x – 3.9759 | 24,510 |
The toxicity of the active ingredients is significant in the development of termite control methods, especially baiting systems. A compound that is toxic but works slowly (slow action) is required in a baiting system, so that the spread of the toxic compound can be widespread (throughout the colony) and then the termite control process can run well. One of the slow-acting materials is eucalyptus leaf extract. Indrayani et al. (2016) stated that eucalyptus leaf extract has a lower termite mortality rate than clove leaf extract. In addition, Adfa et al. (2023) also showed that the n-Hexane extract of Azadirachta excelsa seeds showed slow action on the mortality of subterranean termites, with compounds detected in the n-Hexane extract of A. excelsa seeds in the form of hexadecanoic acid, ethyl ester of the fatty acid esters, 9,12-octadecadienoic acid (Z, Z)-, and 9-octadecenal.
The results of identifying chemical compounds in the base extract of the stems and roots of cajuput seedlings using GC-MS showed that there were 50 types of chemical compounds in the extract, with 20 dominant compounds. Of the several types of dominant compounds, several have bioactive properties (Table 7).
| Retention time | Chemical compounds | Relative concentration (%) | Bioactivity |
|---|---|---|---|
| 21.137 | Hexadecanoic acid (CAS) Palmitic acid | 22.35 | Anti-pest/insect (Zhang et al., 2021), oviposition repellant (Bober and Rafaeli, 2010). |
| 22.387 | 9,12-Octadecadienoic acid (Z,Z)- (CAS) Linoleic acid | 19.25 | Feeding stimulants (Eriksson et al., 2008) and oviposition attractants (Bober and Rafaeli, 2010) |
| 38.626 | Stigmast-5-en-3-ol, oleat | 8.16 | Anti-fungal/fungus (Suryadi et al., 2015), Anti-bacterial (Tayade et al., 2013) |
| 21.644 | 1-Octadecanol (CAS) Stenol | 4.36 | Anti-bacterial, anti-fungal, and anti-larvae (Gnanashree and Sirajudeen, 2018). |
| 25.718 | Cyclotetracosane (CAS) | 3.86 | Anti-microbial and anti-oxidant (Mongalo et al., 2019). |
| 22.943 | 1-Octanol, 2-butyl- (CAS) 2-Butyl-1-octanol | 2.99 | Anti-feedants (Eriksson et al., 2008). |
| 22.746 | (Z,Z)-3,9-cis-6,7-epoxy-nonadecadiene | 2.19 | Sex pheromones (Jing et al., 2019). |
| 23.208 | 2-Pentadecanone, 6,10,14-trimethyl- (CAS) 6,10,14-Trimethyl-2-pentadecano | 1.53 | Anti-bacterial (Filipowicz et al., 2003), anti-fungal/fungus (Radulovic et al., 2011), anti-microbial (Ogunmoye et al., 2020; Radulovic et al., 2011). |
| 22.658 | Tomentosin | 1.35 | Anti-fungal (Mamoci et al., 2011). |
Based on the results of the compound identification, no compounds attracted termites (attractants to termites). However, some compounds act as feeding stimulants and oviposition attractants for insects (9,12-Octadecadienoic acid (Z, Z)- (CAS) Linoleic acid). Eriksson et al. (2008) stated that 9,12-Octadecadienoic acid (Z, Z)- (CAS) Linoleic acid or linoleic acid could act as a feeding stimulant for Hylobius abietis (pine beetle); however, the effect was not significant. Bober and Rafaeli (2010) stated that 9,12- Octadecadienoic acid (Z, Z)- (CAS) Linoleic acid, a free fatty acid, can act as an oviposition attractant. Fatty acids are the major compounds found in the n-Hexane extract of A. excelsa seeds and are thought to affect mortality in the subterranean termite C. curvignathus (Adfa et al., 2023). Conversely, other free fatty acids [Hexadecanoic acid (CAS) and Palmitic acid] act as oviposition repellants. Oviposition is an insect behavior and the last step in insect reproduction. Oviposition is the behavior in which insects lay mature eggs in an external environment (Klowden, 2009).
Linoleic acid is another fatty acid. In addition to its bioactivity against insects (feeding stimulants and oviposition attractants), linoleic acid has other beneficial properties. Muhamad et al. (2017) stated that linoleic acid is a fatty acid that is widely used in cosmetics and is generally used as an emollient or moisturizer for 14 skins, nails, and hair. In addition, Sanders (2016) stated that linoleic acid serves as a water barrier in the skin so that it can hold water well (maintain moisture). Review analisys from Dachev et al. (2021) show various studies from cell and animal models indicate that CLA and individual isomers may have diverse beneficial effects against cancer.
Other dominant compounds found in cajuput seedling root extracts include antibacterial, antimicrobial, and antifungal compounds. Stigmast-5-en-3-ol, an oleic sterol-derived compound classified in the phytoalexin group, has potential as an antifungal (Suryadi et al., 2015). In addition, Mamoci et al. (2011) showed that tomentosin has antifungal activity against Microsporum canis, Microsporum gypseum, and Trichophyton mentagrophytes. (Z, Z)-3, 9-cis-6, 7-epoxy-nonadecadiene is another compound that attracts insects. This compound is found in the sex pheromone of the Ectropis obligua, which distinguishes it from its sister insect Ectropis grisescens.
4. CONCLUSIONS
Cajuput seedling extract solutions at concentrations of 0.75% and 1.00% showed bioactivity as attractants for C. curvignathus termites, with AI values of 0.24 and 0.49. An extract solution with a concentration of 0.75% has the potential to be a termite attractant, because it has a high AI for C. curvignathus termites and is not toxic to them. Linoleic acid, which can act as a feeding stimulant, is one of the dominant compounds in the cajuput seedling root extract.

