1. INTRODUCTION
The genus Litsea, with its diverse range of therapeutically promising plants, has attracted considerable attention because of its wide range of functionalities. Several pharmacological studies have confirmed the traditional medicinal uses of Litsea species. A thorough and systematic investigation of the phytochemical constituents and pharmacological properties of Litsea, especially its mechanisms of action and toxicology, is crucial for understanding its ethnomedicinal uses, exploring its therapeutic potential, and supporting the development of future healthcare products (Wang et al., 2016). Among the most promising Litsea species, Litsea elliptica Blume, a prominent tropical tree native to Southeast Asia, has a long history of medicinal applications. Kuspradini et al. (2018) reported that L. elliptica and L. angulata are the most dominant Litsea species in East Kalimantan, Indonesia. Seven diverse compounds, including flavonoids, alkaloids, and steroids, were previously found in the leaves and wood of L. elliptica extracts (Phoopha et al., 2020). Three major compounds, 2-undecanol (36.35%), terpineol (30.52%), and 9-decen-2-ol (22.43%), were obtained from the essential oil of L. elliptica (Kuspradini et al., 2021). This rich chemical composition contributes to the potential of plants for various applications in medicine and natural product research.
The leaves are particularly revered because of their therapeutic properties against a variety of ailments. Traditional practices often involve the application of crushed leaves to the forehead for headache relief, thereby demonstrating the diverse contributions of the plant to local healthcare. Beyond headaches, L. elliptica leaves are also used to treat stomach ulcers, fever, and asthma, and even repel insects, solidifying their valuable role in healthcare traditions in the region (Goh et al., 2022; Grosvenor et al., 1995; Jiwajinda et al., 2002; Yang et al., 2022a). The young leaves are consumed as a vegetable side dish and used as a flavoring material in Thai ‘Nam Prik,’ a local spicy dip (Ngernsaengsaruay et al., 2011).
In addition to its ecological importance, L. elliptica has garnered attention for its diverse array of bioactive compounds and potential health benefits, including its antioxidant properties (Wong et al., 2014). The discovery of new and safe antioxidants from natural sources holds immense potential for various applications, including natural antioxidants, functional foods, and nutraceuticals. This growing interest has fueled the exploration of plant-based sources of antioxidants, and phytochemical screening has emerged as a valuable method for identifying and investigating these bioactive compounds in plants (Lee et al., 2020; Nkogo et al., 2022). Understanding the chemical composition and antioxidant activities at various ripening stages can assist producers in determining the optimal time for harvesting plants (Um et al., 2020; Yang et al., 2022b). Optimizing the conditions and design parameters is crucial for scaling up the extraction processes. This involves considering factors such as solvent type, particle size, solid-solvent ratio, and temperature, as previously reported by Mindaryani et al. (2023) and Mun et al. (2020).
Previous studies have highlighted the presence of various phenolic compounds in L. elliptica, including flavonoids and tannins, which possess antioxidant properties. However, the influence of leaf maturity and solvent extracts on antioxidant activity remains largely unexplored. This study aimed to address this gap by investigating the variation in the antioxidant activity of L. elliptica leaves and exploring the influence of leaf stage (tender, immature, and mature) and solvent extracts (n-hexane, ethyl acetate, and ethanol) on the antioxidant activity of the extracts. The 2,2’-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) method, a widely recognized and sensitive antioxidant assay, was used in this study to quantify the free radical scavenging capacity of different extract types.
Harvesting L. elliptica leaves at the best stage and extracting them using an appropriate solvent is crucial for enhancing the potential of this plant as a natural source of antioxidants for therapeutic and health-promoting applications. Comprehensive analysis of these factors can provide valuable insights into effective and sustainable extraction strategies and maximize the potential of L. elliptica leaves as a natural source of antioxidants for therapeutic and health-promoting applications.
2. MATERIALS and METHODS
This study was conducted at the Laboratory of Forest Product Chemistry and Renewable Energy and the Laboratory of Forest Cultivation, Faculty of Forestry, Mulawarman University, Samarinda, Brazil. Litsea elliptica leaves were obtained from 6-month-old plants grown in the same faculty. Leaf samples were collected at three stages of maturity: tender, immature, and mature.
After air-drying, the leaf samples were ground into a powder using a mechanical grinder. The extract preparation method was adopted from Kuspradini et al. (2023). Subsequently, maceration was performed using n-hexane, ethyl acetate, and 96% ethanol (technical grade) to extract the powder (10 g). The powder was first extracted with n-hexane for 24 h at a 1:1 (w/v) ratio, and the resulting solution was filtered using filter paper (with a thickness and basic weight specification of 0.0893 m3 and 80 ± 5 g, respectively). Additional extraction was performed with ethyl acetate by soaking the residue in the solvent at a 1:1 (w/v) ratio for 24 h to produce an ethyl acetate extract from the filtrate. Finally, the residue was extracted with ethanol at a 1:1 (w/v) ratio for 24 h, and the resulting filtrate was collected as the ethanol extract. All extractions were performed on a shaker. The filtrate was evaporated using a dehydration machine at 40°C to obtain a crude extract. All extracts (n-hexane, ethyl acetate, and ethanol extracts) were then analyzed using antioxidant assays for total phenolic and flavonoid content.
The phenolic content of the L. elliptica leaf extract was determined according to a previously reported method, with a few modifications (Kuspradini et al., 2016). Briefly, the sample of 100 μL (1 mg of extract in 10 mL aquadest) was mixed with 400 μL aquadest, 250 μL Folin-Ciocalteu reagent, and 1,250 μL of 7.5% sodium carbonate. After 1 h of incubation, the absorbance was measured at 760 nm using a spectrophotometer. Total phenolic content (TPC) was measured using a gallic acid calibration curve and expressed in milligrams of gallic acid equivalent (mg GAE).
A spectrophotometric method (Kuspradini et al., 2016) using a catechin standard was employed with minor modifications. A mixture was prepared by combining 100 μL extract (1 mg/10 mL) with 700 μL aquadest, 100 μL sodium nitrite, 100 μL aluminum chloride, and 500 μL sodium hydroxide. The homogenized mixture was left undisturbed in the dark for 10 min at room temperature. Absorbance was measured at 510 nm using a spectrophotometer. The flavonoid content was calculated using the calibration curve of the catechin standard and presented as milligram catechin equivalents (mg CE).
Antioxidant activity against ABTS radicals was measured according to a previously reported protocol (Toppo et al., 2019). The ABTS solution was prepared by combining a 7.4 mM ABTS solution with a 2.6 mM potassium persulphate solution in a 2:1 ratio. The mixture was incubated for 16 h at room temperature in the dark. The solution was then combined with 1 mL of ABTS solution and 60 mL of methanol. The absorbance of (0.70 ± 0.02) units at a wavelength of 734 nm was measured using a spectrophotometer. Different concentrations (6.25, 12.5, 25, 50, and 100 ppm) of 100 μL of extract were mixed with 900 μL ABTS solution and shaken for 45 s. After 15 min of incubation in the dark, the absorbance of the resulting mixture was measured at 734 nm using a spectrophotometer. Antioxidants were quantified as the percentage of ABTS free-radical scavenging activity. Ascorbic acid (vitamin C) was used as a positive control. The scavenging activity was calculated using the following formula:
The TPC and total flavonoid content (TFC) were calculated using equations based on the linear regression models of gallic acid and catechin, respectively. The models used quercetin concentration as the independent variable (abscissa) and the average absorbance of triplicate measurements at each concentration as the dependent variable (ordinate).
The antioxidant capacity is expressed as the IC50 value, which represents the concentration of the extract required to inhibit 50% of ABTS free radicals. IC50 was calculated using a third-order polynomial regression equation, with the series of extract concentrations and the percentage of ABTS free-radical scavenging activity as the independent (abscissa) and dependent (ordinate) variables, respectively.
3. RESULTS and DISCUSSION
In this study the TPC of L. elliptica leaves showed a significant increase with leaf maturity for all solvent extracts (Fig. 1), similar to report by Wong et al. (2014) whereas, Nobossé et al. (2018) reported a similar increase in Moringa oleifera L. leaves, except for the aqueous extract. These findings suggest that the accumulation of phenolic compounds in leaves is a common phenomenon during plant growth and maturation.
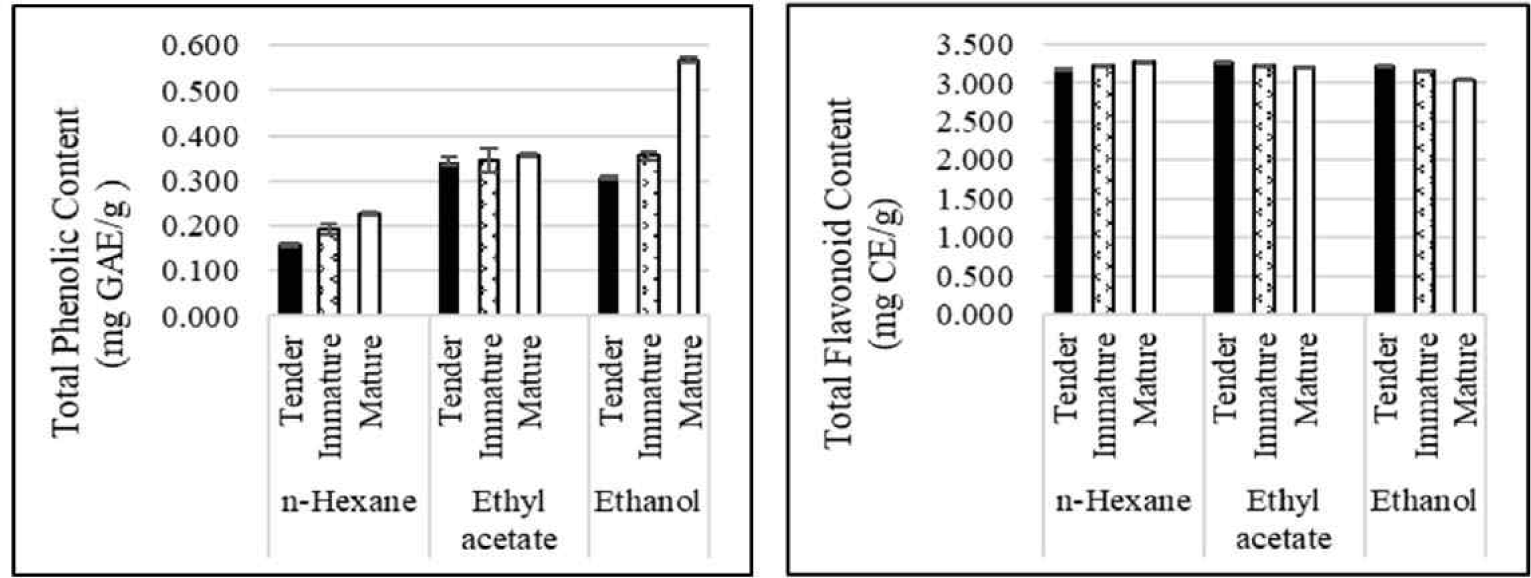
According to Fig. 1 the TPC content of n-hexane extracts, ranged from 0.159 ± 0.003 mg to 0.226 ± 0.003 mg GAE/g extract for tender leaves and 0.194 ± 0.012 mg GAE/g extract for immature leaves to 0.357 ± 0.003 mg GAE/g extract for mature leaves. Similar trends were observed in ethyl acetate and ethanol extracts, with contents ranging from 0.343 ± 0.011 to 0.357 ± 0.03 mg GAE/g extract, 0.343 ± 0.011 to 0.357 ± 0.003 mg GAE/g extract, and 0.307 ± 0.005 to 0.568 ± 0.006 mg GAE/g extract, for tender, immature, and mature leaves, respectively. The results indicated that the TPC in ethanol was substantially higher than that in ethyl acetate and n-hexane. The choice of solvent can significantly affect the extraction yield of phenolic compounds.
This highlights the importance of optimizing the solvent selection to maximize the extraction of the desired bioactive compounds. Variations in the extractable total phenol and flavonoid contents between different Litsea species, including L. elliptica, have been reported (Salehi et al., 2019). This suggests that the specific composition and concentration of phenolic compounds may vary depending on the Litsea species and other factors, such as environmental conditions. The results indicated that the TPC in ethanol was substantially higher than that in ethyl acetate and n-hexane. A separate study (Goh et al., 2022) found that young L. elliptica leaves yielded the most phenolic compounds when extracted with methanol compared to other solvents. This observation confirmed the effectiveness of polar solvents for extracting phenolic compounds from L. elliptica leaves.
The data presented in Fig. 1 show that the TFC in L. elliptica leaves exhibited complex trends depending on both the leaf maturity stage and the solvent extract. This finding highlights the significant interplay between these two factors during flavonoid extraction. Although the TFC increased slightly in the n-hexane extracts with increasing maturity, the difference was minimal. Conversely, the TFC of the ethyl acetate and ethanol extracts decreased from tender to mature leaves, similar to the findings in Morus alba. These findings suggest that the relationship between leaf maturity and flavonoid content may not be universal and may vary depending on the plant species and extraction conditions.
Remarkably, unlike TPC, TFC levels were not dependent on solvent polarity, highlighting the distinct factors that influence the extraction of these two classes of bioactive compounds. Overall, these data reveal the importance of considering both leaf maturity and solvent choice when optimizing extraction protocols to maximize the yield of flavonoids from L. elliptica leaves.
The third-order polynomial regression model accurately predicted the relationship between the concentration and antioxidant activity of L. elliptica, as shown in Fig. 2. The strong correlation coefficient (R2 = 0.9825 to 1) indicated a direct and significant relationship between the extract concentration and antioxidant activity measured by the ABTS assay. This suggests that increasing the concentration of the extract resulted in a proportional increase in antioxidant activity.
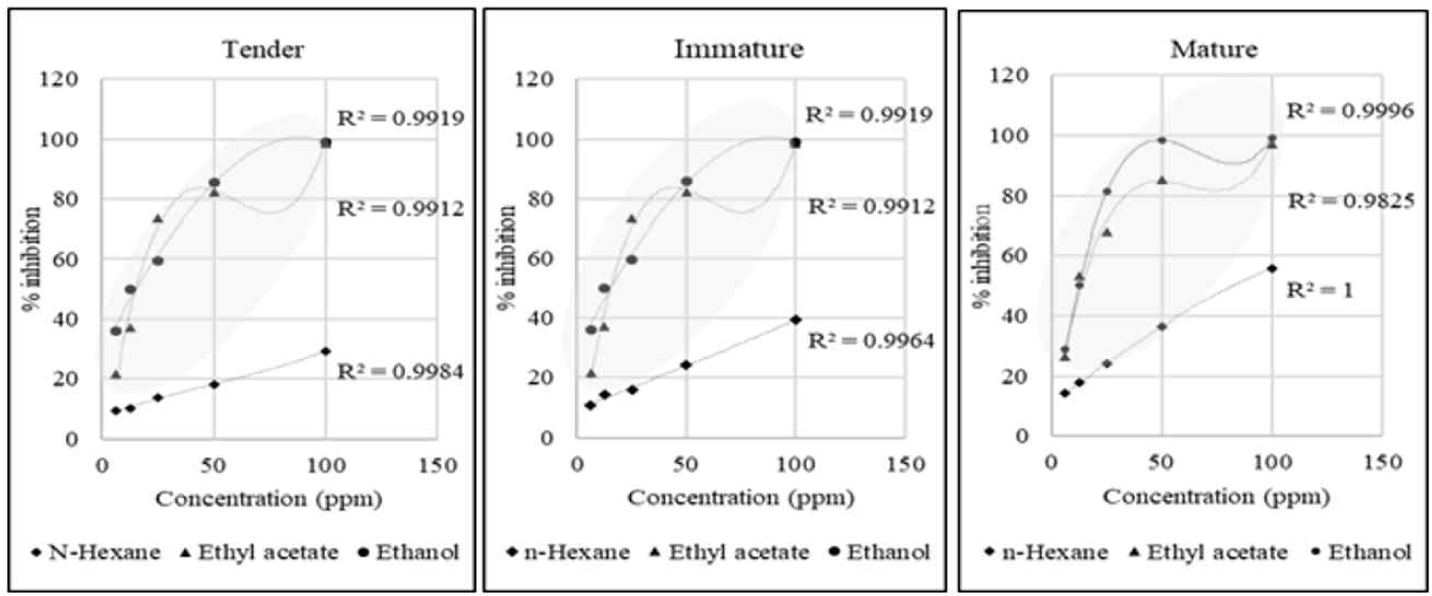
IC50, a widely employed parameter for measuring antioxidant activity, signifies the concentration at which a substance, such as ABTS, inhibits 50% of the free radical activity. Lower IC50 values indicate stronger antioxidant activity. The overall findings in this study suggest that the ethanolic extract from L. elliptica leaves has antioxidant properties ranging from active to highly active (15.34–6.35 ppm). According to Moga’s criteria, IC50 values ≤ 10 μg/mL are highly active, 10–150 μg/ mL are active, 150–500 μg/mL are moderately active, and > 500 μg/mL are inactive (Table 1; Moga et al., 2021).
Highlighting the importance of solvent polarity, the data reveals that ethanol, a highly polar solvent, exhibits the strongest antioxidant activity (Purwanto et al., 2017). This reinforces the observed trend of increasing activity with increasing solvent polarity (Herrera-Pool et al., 2021; Huh et al., 2022).
This emphasizes the importance of selecting an appropriate solvent to maximize the extraction of the desired bioactive compounds, similar to a study that showed appreciably high antioxidant potential of methanolic extracts of L. elliptica leaves (Suksamerkun et al., 2013).
The effect of leaf maturity on antioxidant activity varied across the different solvent extracts. Although tender leaves extracted with ethanol displayed the highest activity, n-hexane and ethyl acetate extracts showed relatively consistent activity across all maturity stages. This suggests that to maximize the antioxidant content, harvesting L. elliptica leaves at the tender stage might be best when using ethanol as the extraction solvent. These findings highlight the potential of L. elliptica leaves as a rich source of natural antioxidants, particularly when extracted with ethanol. The strong correlation between concentration and activity, coupled with the high accuracy of the regression model, provides valuable tools for optimizing the extraction process.
Additionally, a significant correlation was observed between ABTS radical-scavenging activity and leaf age in L. elliptica, with younger leaves showing higher ABTS radical-scavenging activity than older leaves. A similar link was observed in a previous study on A. arguta accessions (Tan et al., 2021).
However, it is important to acknowledge the complex interplay between the factors that influence antioxidant activity. Although a positive correlation between antioxidant activity and total phenol/flavonoid content has been reported (Faiku et al., 2019; Koraqi and Lluga-Rizani, 2022), this relationship is not always straightforward. Factors such as synergistic and antagonistic interactions between different types of phenols and flavonoids, the presence of other bioactive compounds, and the specificity of the ABTS method can contribute to this complexity (Giada, 2013; Hidalgo et al., 2010; Kadum et al., 2019; Munteanu and Apetrei, 2021). Therefore, a comprehensive understanding of these factors is crucial for accurately interpreting the relationship between ABTS activity and phenolic/flavonoid content. A high phenolic/ flavonoid content alone cannot guarantee a strong antioxidant activity.
Additional investigations exploring the specific compounds responsible for the observed antioxidant activity and the potential applications of L. elliptica extracts in various fields are necessary to fully elucidate the potential of this promising natural resource.
4. CONCLUSIONS
The data presented in this study confirmed the influence of leaf maturity and solvent selection on the extraction of phenolic compounds and flavonoids from L. elliptica leaves. These findings underscore the importance of considering these factors when optimizing extraction protocols to maximize the yield of bioactive compounds with potential health benefits. These data suggest that ethanol is the most effective solvent for extracting phenols from mature L. elliptica leaves.
These results suggest that polar solvents are suitable for the extraction of antioxidant compounds from plant materials, particularly from L. elliptica. Furthermore, harvesting tender leaves may improve the antioxidant content when ethanol is used as the extraction solvent. It is important to note that the antioxidant activity of L. elliptica leaf extract can be influenced by antioxidant compounds other than phenols and flavonoids.
Further research is required to explore the specific factors contributing to the observed differences in antioxidant activity between leaves of different ages. This could involve analyzing the changes in individual phenol and flavonoid profiles and investigating the potential influence of other bioactive compounds present in the leaves.








