1. INTRODUCTION
Indonesia’s tropical forests are rich in diverse flora. Approximately 4,000 wood species have been identified in these forests (Ariyanti et al., 2016). Among these, 85% are classified as having low durability, while only 15% are considered durable. The low durability of wood increases its vulnerability to wood-destroying organisms such as termites, wood rot fungi, and other wood borers (Arinana et al., 2022; Ariyanti et al., 2016; Hutabarat et al., 2019; Luth, 2020).
Termites can damage buildings and wood products, causing economic losses. The financial impact attributed to termite activity surpasses that caused by fires, storms, or floods (Nandika et al., 2015). Enhancing the resistance of wood to degradation by organisms, namely, subterranean termites, using preservation methods can mitigate these losses (Daviyana et al., 2013; Hutabarat et al., 2019).
Tamanu (Calophyllum inophyllum L.) is a tropical plant found in several coastal areas of Indonesia. C. inophyllum is a rich source of biologically active secondary metabolites (Ee et al., 2011). Studies have reported that extracts from the leaves and bark of C. inophyllum possess antitumor, analgesic (Sundur et al., 2014), antiarthritic, anti-HIV, antirheumatic (Perumal et al., 2016), and antibacterial properties (Mishra et al., 2010; Sundur et al., 2014). Extracts derived from the bark of C. inophyllum exhibit antifungal activities (Ee et al., 2011), while those obtained from its fruits and seeds demonstrate anti-inflammatory effects (Ee et al., 2011; Zakaria et al., 2014). However, the anti-termite activity of extracts from the stem bark of C. inophyllum has not been assessed. Utami and Haneda (2012) reported that C. inophyllum oil is effective against insect pests such as Pteroma plagiophleps Hampson (bagworms), which attacks the leaves and bark of forest onions (Scorodocarpus borneensis). Further, Ee et al. (2011) demonstrated that phytochemical extracts isolated from the stem bark of C. inophyllum contain different types of quinones. Quinones are a group of phenolic compounds that are toxic to wood-destroying organisms, including termites and wood-rotting fungi. These studies suggest that the stem bark extract of C. inophyllum is a potential wood preservative capable of inhibiting the growth of wood-destroying organisms, specifically, subterranean termites. Therefore, this study focused on the extractive analysis and activity of the bark extract of C. inophyllum as a wood preservative against subterranean termites. Furthermore, it aimed to analyze the anti-termite properties and identify the active compounds within the C. inophyllum bark extract that exhibit toxicity toward subterranean termites.
2. MATERIALS and METHODS
C. inophyllum bark samples were obtained from 35-year-old logs with a 32 cm diameter in Penedagandor Village, East Lombok Regency, West Nusa Tenggara, Indonesia. The chemicals used for extraction included n-hexane, ethyl acetate, and methanol, all sourced from Merck. Further, WhatmanTM Cytiva cellulose paper No. 1 (Cat No. 1001-125, Whatman, Maidstone, UK), acetone (Merck, Darmstadt, Germany), and the subterranean termite, Coptotermes curvignathus (C.curvignathus) Holmgren, were utilized in the bioassay.
Approximately 1,500 g of C. inophyllum stem bark powder (40–60 mesh) was extracted with different solvents, including n-hexane, ethyl acetate, and methanol. The maceration method was employed for extraction (Vittaya et al., 2023), with a powder-to-solvent ratio (based on dry weight) of 1:4 (w/v). Each extraction process was performed for 48 h and repeated until a clear solution was obtained. Subsequently, the obtained n-hexane, ethyl acetate, and methanol extracts were evaporated using a vacuum rotary evaporator at a maximum temperature of 60°C until the solvent was completely removed. The yield of each extract was then calculated using Equation (1) (Ella Nkogo et al., 2022).
Where
R = yield of extract (%).
Mext = mass of the extract after evaporation (g).
Mech = initial mass of the bark powder sample (g).
A modified paper disc method was employed for the termite bioassay (Mukai et al., 2017). Each powder extract from n-hexane, ethyl acetate, and methanol was dissolved in acetone at concentrations of 4%, 6%, 8%, and 10% (w/v). The test was conducted using the no- and two-choice methods. In the no-choice test, one paper disc sample was placed inside an acrylic tube measuring 8 cm in diameter and 6 cm in height. Two control paper disc samples were used: one without any treatment and another treated with acetone. Each test paper was dripped with n-hexane, ethyl acetate, and methanol extracts at concentrations of 4%, 6%, 8%, and 10% (w/v). Subsequently, the test paper was placed in an acrylic tube, following which 45 worker and 5 soldier termites were added. The details of the treatments for the paper disc samples in the no-choice method are presented in Table 1.
| Treatments | Concentration extract in acetone (w/v, %) | |
|---|---|---|
| Control | C (without treatment) | - |
| 0% (with acetone only) | 0 | |
| Extracts | n-Hexane | 4 |
| 6 | ||
| 8 | ||
| 10 | ||
| Ethyl acetate | 4 | |
| 6 | ||
| 8 | ||
| 10 | ||
| Methanol | 4 | |
| 6 | ||
| 8 | ||
| 10 |
In the two-choice test, a single control was used, consisting of a test paper without any treatment. This test was performed following the method described by Mukai et al. (2017), with some modifications. Two test papers were placed inside each test bottle, with one serving as the control and the other as the treated paper with 4%, 6%, 8%, and 10% extract solutions. Subsequently, 90 worker and 10 soldier termites of C. curvignathus were introduced into each test bottle. The anti-feedant properties of the C. inophyllum extract against subterranean termites were analyzed using the two-choice test. The test bottles for the no- and two-choice tests were placed in a dark room at room temperature (28 ± 2°C) and maintained at 80% ± 5% relative humidity for 7 d (Adfa et al., 2020, 2023).
In this bioassay, termite mortality and the weight loss of the test paper were evaluated. Termite mortality was determined on the seventh day of the test using Equation (2) (Arsyad et al., 2020).
Where
T1 = initial number of termites.
T2 = the number of live termites after the test.
The weight loss of the samples was calculated on the 7th day using Equation (3) (Hadi et al., 2018).
Where
W1 = weight of the oven-dried sample before testing (g).
W2 = weight of the oven-dried sample after testing (g).
The active compound present in the C. inophyllum stem bark extract, which exhibited the most promising termite-resistant properties, was identified using gas chromatography–mass spectrometry (GC-MS). A total of 6 μL of the extract solution was added to the inlet for analysis. Data processing was performed using GC-MS data analysis software. The GC-MS data were collected with a GCMS-QP 2010 (Shimadzu, Kyoto, Japan) instrument, following the method outlined by Oramahi et al. (2022). A Stabilwax-DA Capillary Column with a 0.25 mm diameter and 30 m length was employed. The injection temperature was set to 250°C, while the column temperature ranged from 60°C to 200°C, with a temperature increase rate of 10°C/min. Additionally, a helium flow rate of 40.0 mL/min was maintained throughout the analysis. GC-MS experiments were conducted in electron ionization mode at 70 eV, with an interface temperature of 200°C. Compounds were identified by comparing mass spectral data with information available in the WILEY 9th library. Further, chemical component analysis was performed according to the method described by Schauer et al. (2005).
The obtained data were statistically analyzed using analysis of variance in a completely randomized factorial design. The objective was to determine the effect of the combination of factor A (solvent type: n-hexane, ethyl acetate, and methanol) and factor B (extract concentrations: 4%, 6%, 8%, and 10%) on the weight loss of the test paper and termite mortality using SPSS 25.0. In cases where the treatment exhibited a significant effect, further tests were conducted using Duncan’s test at α = 5%.
3. RESULTS and DISCUSSION
The yield of the extract varied, as shown in Table 2. This disparity in extract yield was attributed to differences in solvent polarity and the types of compounds present in the extracts (Lukmandaru, 2012; Rohmah et al., 2020; Yanti et al., 2012). The highest extract yield was observed with methanol, indicating that the stem bark extract of C. inophyllum tended to be polar. Methanol, being more polar than n-hexane and ethyl acetate, dissolves polar compounds. Conversely, n-hexane dissolves nonpolar compounds, while ethyl acetate dissolves semipolar components (Lukmandaru, 2012). The methanol extract displayed a dark brown color, ethyl acetate extract exhibited a lighter brown color than did the methanol extract, and n-hexane extract appeared yellow. Furthermore, the yield of the n-hexane extract surpassed that of the ethyl acetate extract, consistent with the results reported by Ee et al. (2011), who observed a higher production of the n-hexane extract from the bark of C. inophyllum compared with that of the ethyl acetate extract. Kadir et al. (2015) reported that the yield of the methanol extract from C. inophyllum heartwood was higher than those of other solvents. In addition, Vittaya et al. (2023) observed that the yield of the methanol extract from C. inophyllum flowers exceeded those of n-hexane and ethyl acetate extracts.
| Soluble extract | Weight of extract powder (g) | Yield (%) |
|---|---|---|
| n-Hexane | 125.12 ± 1.15 | 8.34 ± 0.06 |
| Ethyl acetate | 69.79 ± 2.77 | 4.65 ± 0.19 |
| Methanol | 258.71 ± 5.08 | 17.25 ± 0.46 |
Table 2 shows that the total bark extract of C. inophyllum was 30.24%. Based on these results, the C. inophyllum stem bark extract was classified into a high extractive substance class according to the Indonesian hardwood chemical component classification. If the extract content exceeded 4%, it was categorized as the high-component class of extractive substances (Jasni et al., 2016). The results of this study align with those of Perumal et al. (2016), who reported that the extract yield from C. inophyllum stem bark was 24.14%, while that from C. inophyllum seeds was 41.5%.
The mortality of C. curvignathus termites served as an indicator of the anti-termite activity of the extracts. A high mortality value indicates stronger anti-termite activity of the extracts. The termite mortality values are depicted in Fig. 1. The extracts of n-hexane, ethyl acetate, and methanol resulted in mortality of the subterranean termite C. curvignathus, reaching up to 75.33% within 7 d of testing. As illustrated in Fig. 1, termite mortality increased with higher extract concentrations applied to the test paper. The mortality of termites on test papers treated with the extracts surpassed that of termites on the control papers, including both the untreated control (C) and the control treated solely with acetone (0%). The success of this test was indicated by termite mortality in the controls remaining below 55% (Harsono, 2016). The mortality of termites in the control group was caused by their inability to adapt to the new environment and the absence of alternative food sources apart from the test paper (Rislyana et al., 2015). Thus, the results showed that C. inophyllum extract demonstrated anti-termite activity, as evidenced by the high termite mortality observed in each treatment of the C. inophyllum extract.
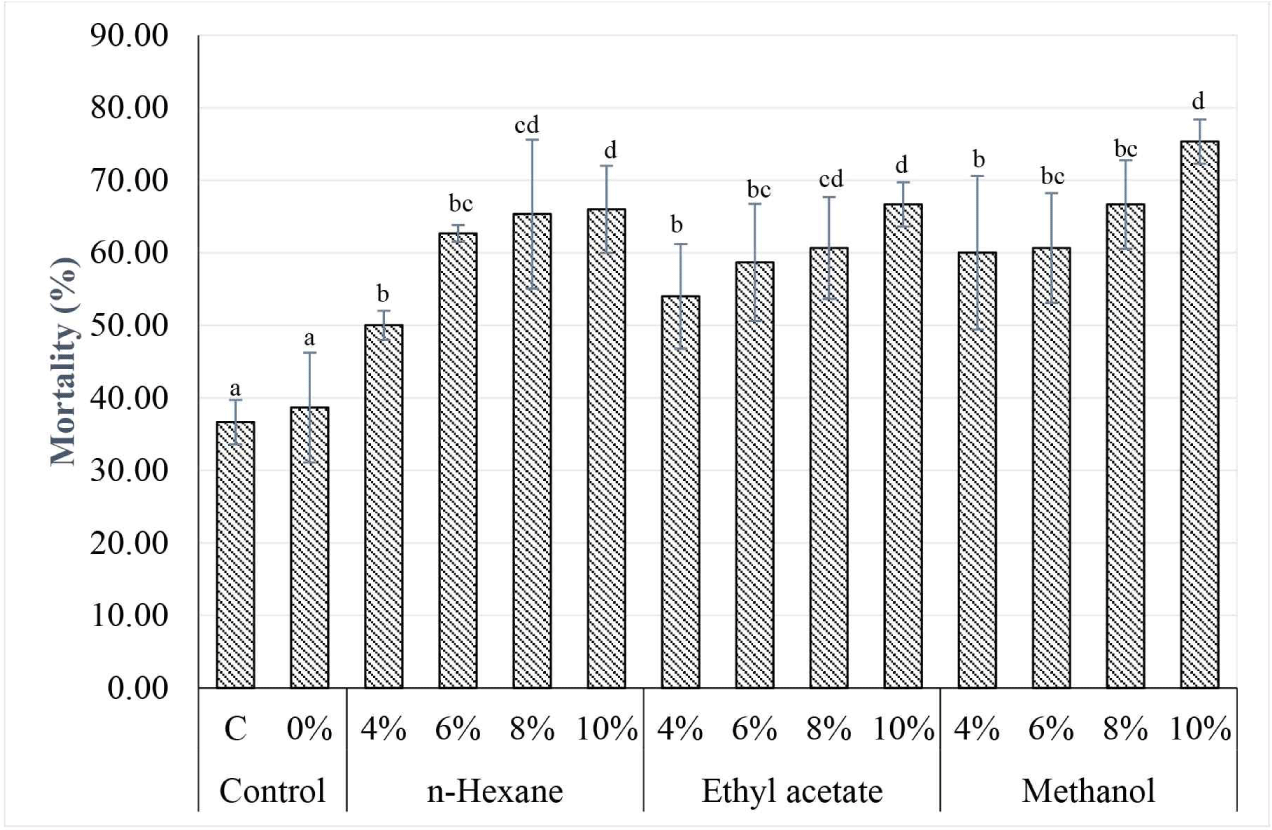
The analysis of variance revealed that the concentrations of the three extracts significantly affected subterranean termite mortality. By contrast, the extract type did not have a significant effect on subterranean termite mortality. These results suggested that C. inophyllum extracts are toxic to subterranean termites. Each solvent was capable of extracting bioactive compounds from C. inophyllum extracts that exhibited toxicity against the subterranean termite C. curvignathus. Further analysis using Duncan’s test indicated that all concentrations (4%, 6%, 8%, and 10%) of the extracts lead to higher subterranean termite mortality compared to that of the control group. Based on the mortality values observed in this test, the most effective concentration for controlling subterranean termites was determined to be 10% for all three extracts used.
The weight loss of the test paper indicates the consumption rate of subterranean termites on the test paper. A higher weight loss suggests lower anti-termite properties of the test paper. The average weight loss of the test paper, as shown in Fig. 2, varies greatly depending on the solvent type and concentration of the extract applied to the test paper. The weight loss of the control test paper was significantly greater than that of the treated test paper. This difference was ascribed to the higher consumption of the test paper by termites in the control group. It was evident that the weight loss of the treated test paper with C. inophyllum extract decreased with increasing concentrations of the extract on the test paper, except for the 6% ethyl acetate extract. The reduction in the weight loss of the test paper as a result of increasing extract concentration showed that the addition of extract provides resistance to the test paper against subterranean termite attack (Septiana and Husni, 2017). The highest weight loss occurred in the control group, while the lowest weight loss was observed in the test paper treated with 10% n-hexane and 10% ethyl acetate. This suggested that the n-hexane and ethyl acetate extracts applied to the test paper contained a substantial amount of toxic compounds. Consequently, the increased reluctance of termites to consume the test paper led to higher termite mortality.

Additionally, the analysis of variance revealed that the interaction between the types and concentrations of extracts considerably influenced the weight loss of the test paper. Duncan’s test further showed that all treatments involving the application of extracts to the test paper resulted in lower weight loss values compared to both the untreated control (C) and control treated with acetone (0%). The treatment with n-hexane extract at concentrations of 6%–10% resulted in weight loss that was not significantly different, whereas the concentration of 4% differed significantly from the weight loss observed at concentrations of 6%–10%. Conversely, for the treatment with ethyl acetate extract, varying the concentration did not lead to significantly different weight loss values. However, the addition of 4%–6% concentrations during the treatment of methanol resulted in substantially different weight loss results compared to concentrations of 8%–10%.
Another indicator used to assess the activity of extractive substances is their anti-feedant value, which was determined through the two-choice test. If the decrease in weight of the treated test paper is smaller than that of the control, a higher anti-feedant value is suggested, indicating that the extractive substance acts as an effective feeding inhibitor for subterranean termites. The average weight of termite consumption of the test paper in the two-choice test is listed in Table 3.
Table 3 demonstrates that the test paper treated with C. inophyllum stem bark extract experienced considerably lower weight loss than the control. This indicates that the bark extract of C. inophyllum functioned as an anti-feedant, inhibiting the feeding activity of subterranean termites. Hence, termites showed a preference for consuming untreated paper over test paper treated with C. inophyllum bark extract. The analysis of variance further displayed that the concentration of the extract solution of C. inophyllum stem bark had a significant effect on the termite consumption of the test paper. In contrast, the type of extract solution had no notable effect on the termite consumption of the test paper treated with the extract.
The active compounds were identified in the n-hexane and ethyl acetate extracts, which yielded the most effective results in controlling C. curvignathus subterranean termites. The results of the GC-MS analysis revealed that the n-hexane extract of C. inophyllum stem bark contained 89 compounds, while the ethyl acetate extract contained 84 compounds. Table 4 presents the ten main components of the most active compounds, identified based on the highest similarity index and area (%) of the n-hexane extract of C. inophyllum stem bark. Generally, n-hexane extract contains compounds predominantly composed of alkaline compounds, glycerol, fatty acids, fatty acid esters, and resin acids (Adfa et al., 2023; Lukmandaru, 2012). This corresponds with research results showing that the n-hexane extract from the extractive substance of C. inophyllum stem bark contains compounds belonging to the glycerol, ester, and fatty acid groups. Similarly, Vittaya et al. (2023) reported that the n-hexane extract derived from C. inophyllum flowers was primarily composed of compounds from the ester, fatty acid, and aromatic groups.
Furthermore, Table 4 shows that the compound with the highest area in the n-hexane extract of C. inophyllum stem bark is friedelan-3-one (4.96%), which belongs to the terpene compounds. Vittaya et al. (2023) noted that the n-hexane extract obtained from C. inophyllum flowers comprises terpene compounds like monoterpenes, sesquiterpenes, and triterpenes. Several terpene compounds, including those from the monoterpene and sesquiterpene classes, have been reported to possess anti-termite activity (Meisyara et al., 2021). Friedelan-3-one has been associated with various biological activities. Odeh et al. (2016) documented its antimicrobial activity, suggesting its potential to inhibit various microorganisms. This leads to the speculation that friedelan-3-one may exhibit anti-termite properties. Additionally, the GC-MS analysis detected alkanes and lipids in the n-hexane extract of C. inophyllum stem bark.
Table 5 outlines the ten primary components of the compounds with the highest similarity index and area (%) found in the ethyl acetate extract of C. inophyllum stem bark. The results of the GC-MS analysis indicated that the ethyl acetate extract of C. inophyllum stem bark was dominated by alcohols, esters, aromatic organic compounds, and fatty acids. Similarly, Vittaya et al. (2023) reported that the ethyl acetate extract from C. inophyllum flowers contained alcohols, alkanes, esters, fatty acids, and aromatic compounds. The compound with the highest area (%) found in the ethyl acetate extract of C. inophyllum stem bark was 1,2-benzene dicarboxylic acid, dinonyl ester (5.44%), which belongs to a class of ester compounds. Rahman and Anwar (2006) reported that the 1,2-benzene dicarboxylic acid, dinonyl ester contained in Plumbago zeylanic extract possessed antifungal activity and could inhibit other organisms, suggesting its anti-termite properties. In addition, friedelan-3-one was also identified in the ethyl acetate extract. This compound belongs to the same class as the compound found in the n-hexane extract, which is believed to exhibit anti-termite activity in the ethyl acetate extract of C. inophyllum stem bark. According to Suprianto et al. (2023), the anti-termite activity of wood stem bark extract may be attributed to its chemical components, including acetic acid, propanoic acid, phenol, and phenol derivatives.
4. CONCLUSIONS
C. inophyllum bark is rich in extractive substances, particularly stem bark extract, which exhibits strong anti-termite and anti-feedant properties against the subterranean termite C. curvignathus. All types of extracts dissolved in different solvents showed termiticidal properties, with efficacy depending on the concentration applied to the test paper. A higher extract concentration resulted in increased termite mortality and reduced weight loss of the test paper. The n-hexane extract of C. inophyllum stem bark contained friedelan-3-one, which possesses anti-termite properties. Similarly, the ethyl acetate extract contained 1,2-benzene dicarboxylic acid, dinonyl ester, and friedelan-3-one, which also exhibit anti-termite properties. Further research is warranted to explore the potential use of the extractive components of C. inophyllum stem bark for controlling dry wood termites and wood decay fungi.








